Supramolecular Chemistry
Supramolecular Chemistry
For the construction of molecular assemblies and materials of higher hierarchical order, we systematically employed non-covalent binding motifs such as hydrogen bonding, electrostatic bonding and hydrophobic interactions. For this purpose we design and synthesize new molecules, which carry the required recognition motifs in their periphery. Examples are precisely defined oligo-electrolytes with up 60 charges in their periphery or porpyrines, perylenes, fullerenes and carbon nanotubes that involve Hamilton receptor/cyanuric acid binding motifs. Of practical use are in particular layer-by-layer assembly architectures where positively and negatively charged chromophores are successively deposited on surfaces. In this way for example new photovoltaic devices can be designed.
Selected Publications
- , , , , , , , , , , , :
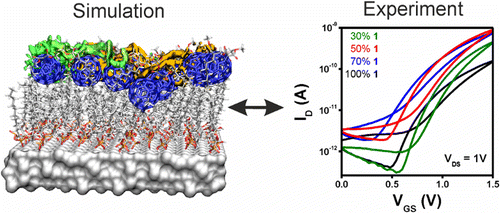
Improving the Charge Transport in Self-Assembled Monolayer Field-Effect Transistors: From Theory to Devices
In: Journal of the American Chemical Society 135 (2013), p. 4893-4900
ISSN: 0002-7863
DOI: 10.1021/ja401320n
A three-pronged approach has been used to design rational improvements in self-assembled monolayer field-effect transistors: classical molecular dynamics (MD) simulations to investigate atomistic structure, large-scale quantum mechanical (QM) calculations for electronic properties, and device fabrication and characterization as the ultimate goal. The MD simulations reveal the effect of using two-component monolayers to achieve intact dielectric insulating layers and a well-defined semiconductor channel. The QM calculations identify improved conduction paths in the monolayers that consist of an optimum mixing ratio of the components. These results have been used both to confirm the predictions of the calculations and to optimize real devices. Monolayers were characterized with X-ray reflectivity measurements and by electronic characterization of complete devices.
- , , , :
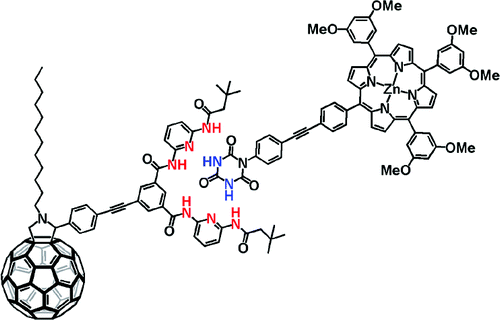
Pairing fullerenes and porphyrins: Supramolecular wires that exhibit charge transfer activity
In: Journal of the American Chemical Society 132 (2010), p. 10786-10795
ISSN: 0002-7863
DOI: 10.1021/ja101937w
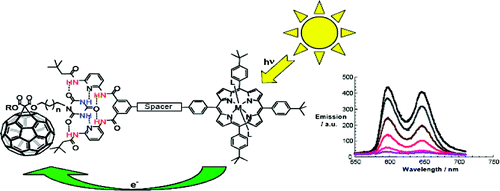
A concept is elaborated of pairing electron donors and electron acceptors that share a common trait, wire-like features, as a powerful means to realize a new and versatile class of electron donor−acceptor nanohybrids. Important variables are fine-tuning (i) the complexation strength, (ii) the electron/energy transfer behavior, and (iii) the solubilities of the resulting architectures. In particular, a series of supramolecular porphyrin/fullerene hybrids assembled by the hydrogen bonding of Hamilton receptor/cyanuric acid motif has been realized. Putting the aforementioned variables into action, the association constants (Kass), as they were determined from 1H NMR and steady-state fluorescence assays, were successfully tweaked with values in the range of 104−105 M−1.
- , , , , , :
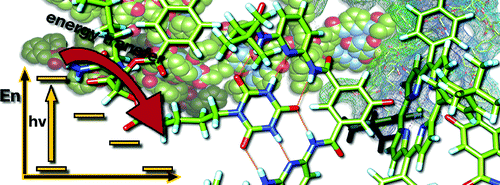
Cooperativity and tunable excited state deactivation: Modular self-assembly of depsipeptide dendrons on a Hamilton receptor modified porphyrin platform
In: Journal of the American Chemical Society 130 (2008), p. 8491-8501
ISSN: 0002-7863
DOI: 10.1021/ja8018065
A series of novel supramolecular architectures were built around a tin tetraphenyl porphyrin platform 6—functionalized by a 2-fold 1-ethyl-3-3-(3-dimethylaminopropyl)carbodiimide (EDC) promoted condensation reaction—and chiral depsipeptide dendrons of different generations 1—4. Here, implementation of a Hamilton receptor provided the necessary means to keep the constituents together via strong hydrogen bonding. Characterization of all architectures has been performed, including 4 which is the fourth generation, on the basis of NMR and photophysical methods. In particular, several titration experiments were conducted suggesting positive cooperativity, an assessment that is based on association constants that tend to be higher for the second binding step than for the first step. Importantly, molecular modeling calculations reveal a significant deaggregation of the intermolecular network of 6 during the course of the first binding step. As a consequence, an improved accessibility of the second Hamilton receptor unit in 6 emerges and, in turn, facilitates the higher association constants. The features of the equilibrium, that is, the dynamic exchange of depsipeptide dendrons 1−4 with fullerene 5, was tested in photophysical reference experiments. These steady-state and time-resolved measurements showed the tunable excited-state deactivations of these complexes upon photoexcitation.
- , , , , , , :
Implementation of a Hamiliton-receptor-based hydrogen-bonding motif toward a new electron donor-acceptor prototype: Electron versus energy transfer
In: Journal of the American Chemical Society 129 (2007), p. 16057-16071
ISSN: 0002-7863
DOI: 10.1021/ja075751g
A new modular concept for the self-assembly of electron donor−acceptor complexes is presented that ensures (i) fine-tuning the strength of the complexation, (ii) controlling the electronic coupling to impact electron and energy transfer processes, and (iii) high solubility of the corresponding hybrid architectures. This task has been realized through developing a series of porphyrin−fullerene donor−acceptor systems held together by a Hamilton-receptor-based hydrogen-bonding motif. In this context, novel libraries of C60 monoadducts (1) containing cyanuric acid side chains and of tetraphenylporphyrin derivatives (2) involving the complementary Hamilton-receptor unit were synthesized.
- , , , , :
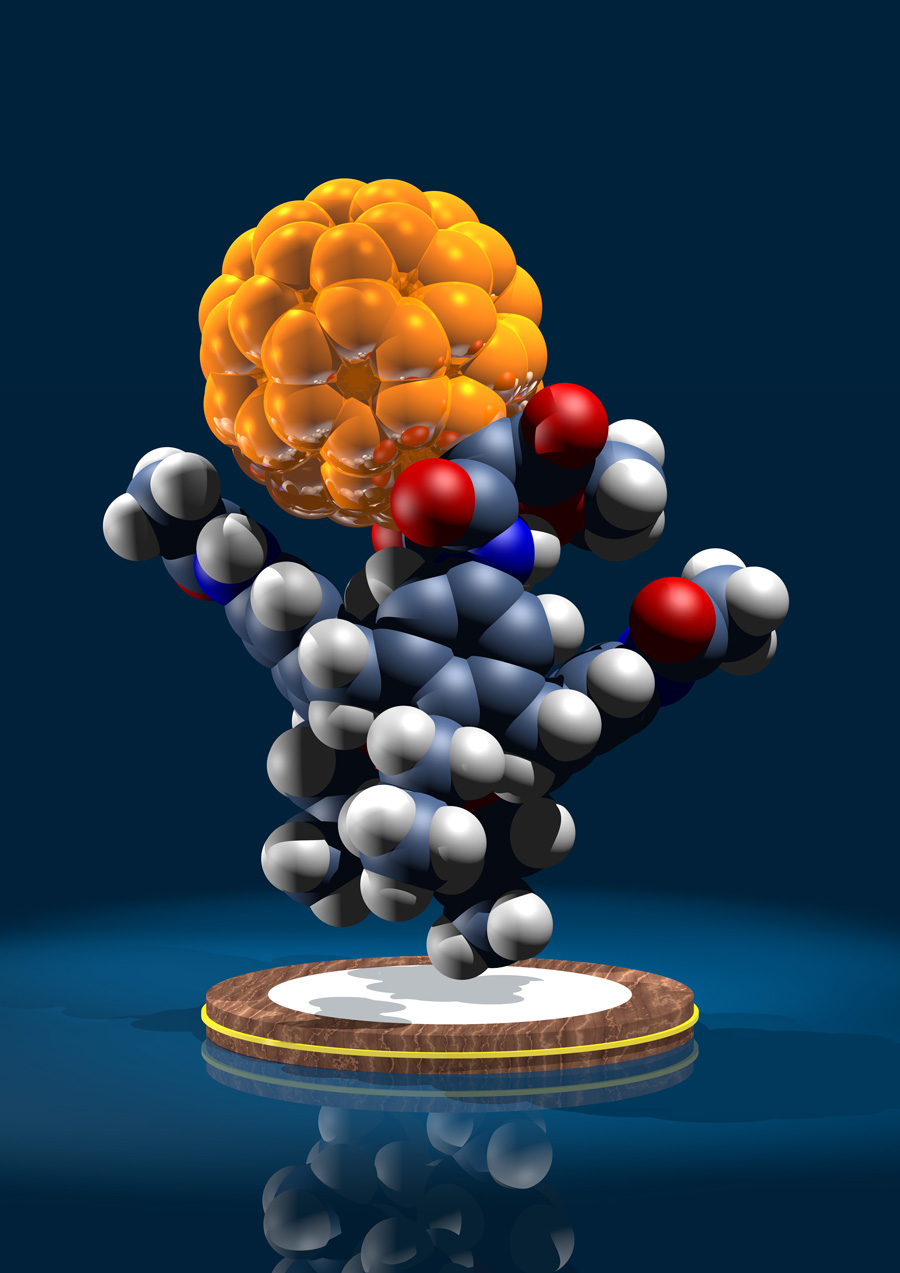
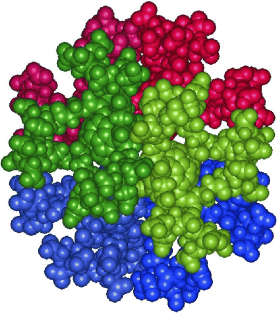
Supramolecular structure of 5-nm spherical micelles with D3 symmetry assembled from amphiphilic [3:3]-hexakis adducts of C60
In: Angewandte Chemie International Edition 46 (2007), p. 4393-4396
ISSN: 1433-7851
DOI: 10.1002/anie.200604784
Six molecules of an amphiphilic fullerene derivative make up the smallest persistent micelle detected so far (see picture, the six amphiphilic molecules are shown in different colors). These micelles can be used to systematically study the factors that determine the structural persistence of micelles and may lead to the design of tailor-made supramolecular containers.
- , , , , :
Switchable supramolecular organization of structurally defined micelles based on an amphiphilic fullerene
In: Angewandte Chemie International Edition 44 (2005), p. 2976-2979
ISSN: 1433-7851
DOI: 10.1002/anie.200462465